Some Examples of Our Research are Explained Below:
Impedance Spectroscopy Measurements and Other ill-posed Inverse Problems; ISGP
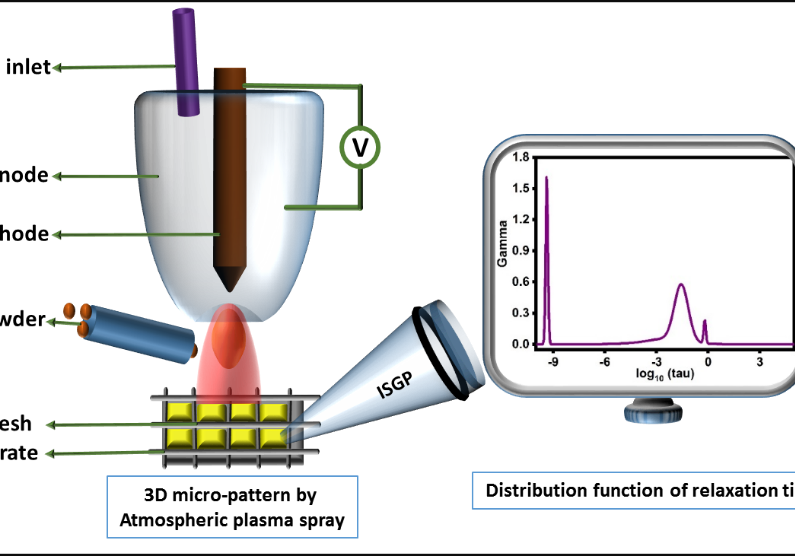
One of our main tools for analyzing electrical properties is impedance spectroscopy. The analysis of impedance spectroscopy data is an ill-posed inverse problem. We have developed a new approach for dealing with the data, taking advantage of the pre-knowledge about the system. ISGP is a program that we have developed that finds an analytical form of the underlying distribution function of relaxation times (DFRT; a.k.a. DRT by some other authors). This allows one to separate different processes and study their responses to environmental changes, giving a clear advantage over deconvolution methods. The DFRT approach in general, and ISGP in particular, are being considered superior to older analysis procedures such as equivalent circuits by increasing number of researchers in the field. We have applied it to research on many systems including fuel cells, super-capacitors, mixed ionic-electronic conductors, etc. See the publication list for references.
We have also applied our new approach to analysis of other inverse problems, notably scatterometry of deep UV light for line edge roughness detection. This was done in cooperation with Nova Ltd. Our new approach makes it possible to put a relatively cheap machine in-line and detect all the wafers at a rate of seconds per wafer.
Defect Chemistry of Perovskites
Here we were involved in understanding the role of metal vacancies in barium titanate and revisiting accordingly the subject of defect chemistry of barium titanate and similar materials. We were also involved in finding the trends of site occupancy of trivalent dopants in barium titanate, and explaining the role of some of these dopants in life-time improvement of capacitors. We have demonstrated the importance of oxygen activity in the very early sintering of nanopowders. We have introduced the concept of doping factor, which allows one to calculate the changes in concentrations of all the native defects as a result of doping in the dilute limit. While the concept of doping factor has been initially developed for binary oxides, we have recently expanded it to ternary oxides, see for instance our feature article in journal of electroceramics, 2014, titled: The correlation between non-stoichiometry and charge compensation in perovskites.
Synthesis of Nano-Ceramic Materials
We learn and develop synthesis routes to obtain and stabilize nanopowders, in particular of ceramic materials. This includes hydrothermal synthesis of barium titanate (pure and doped); hydrolysis of titania and titania-niobia solid solutions; hydrolysis of zinc peroxide nanoparticles and production of zinc oxide nanoparticles; combustion synthesis of perovskites and of silver embedded in carbon nanoparticles. It is important to produce the nanopowders that we investigate in our lab, since nanopowders are typically systems that are not in thermodynamic equilibrium, and hence their properties are strongly influenced by the synthesis route, on which we would like to have control. Great deal of our efforts in this area is directed to the development of simple, inexpensive and preferably water based routes. We have demonstrated EPD of barium titanate nanopowder that we synthesized and stabilized in our lab to create thin films of sintered barium titanate on Ni electrodes by cathodic water-based electrophoretic deposition. We use simple methods to make ReRAMs of thin layers of titanium oxide between similar or dissimilar electrodes. We prepare nano ceramic inks for printing.
Energy and Environmental Science
Electrochemical water splitting
We learn and develop synthesis routes to obtain and stabilize nanopowders, in particular of ceramic materials. This includes hydrothermal synthesis of barium titanate (pure and doped); hydrolysis of titania and titania-niobia solid solutions; hydrolysis of zinc peroxide nanoparticles and production of zinc oxide nanoparticles; combustion synthesis of perovskites and of silver embedded in carbon nanoparticles. It is important to produce the nanopowders that we investigate in our lab, since nanopowders are typically systems that are not in thermodynamic equilibrium, and hence their properties are strongly influenced by the synthesis route, on which we would like to have control. Great deal of our efforts in this area is directed to the development of simple, inexpensive and preferably water based routes. We have demonstrated EPD of barium titanate nanopowder that we synthesized and stabilized in our lab to create thin films of sintered barium titanate on Ni electrodes by cathodic water-based electrophoretic deposition. We use simple methods to make ReRAMs of thin layers of titanium oxide between similar or dissimilar electrodes. We prepare nano ceramic inks for printing.
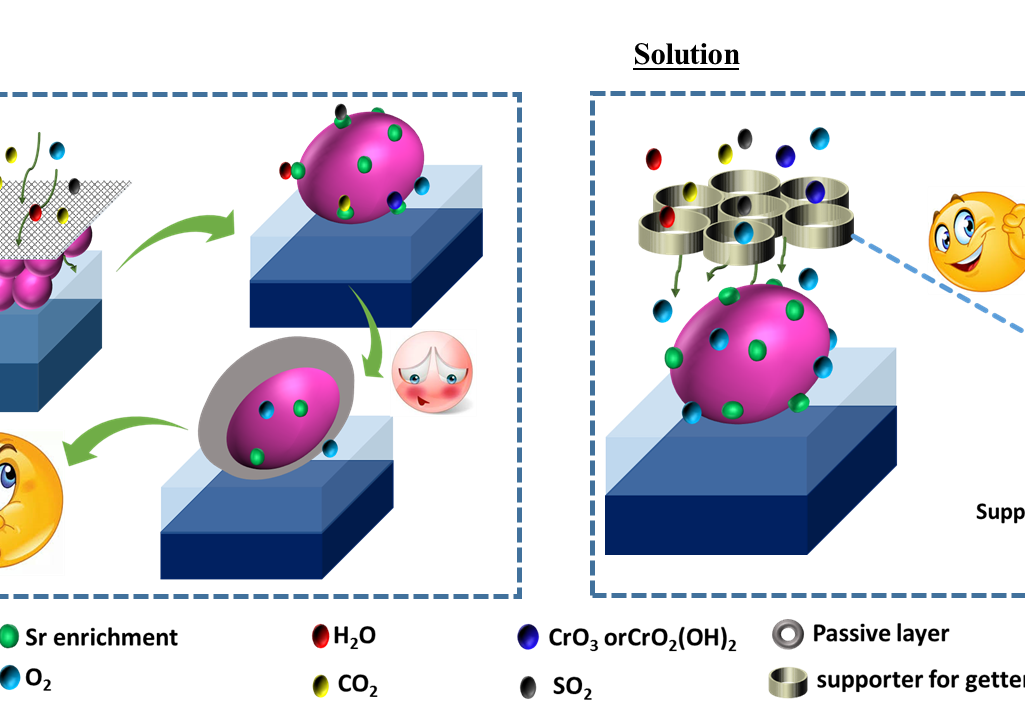
Mitigation of cathode poisoning in solid oxide fuel cells (SOFC)
Cathode poisoning in SOFC is a major issue that limits the device durability. Recently, the getter is an emerging idea to mitigate cathode poisoning. Using the merit of this idea, here, we have developed 3D CaO.xAl2O3 foam using cheap starting precursors of whole egg and Al2O3. The egg white has made by CaCO3, which used as the starting precursor for CaO. Likewise, the egg white and yolk helps to form 3D foam of CaO.Al2O3. CaO.Al2O3 is an efficient getter material to capture the impurities from the air and interconnects that revealed from Thermodynamic calculation. The foam displays more inter-particle spaces, which encourages the easy flow of gaseous and improves the interaction to getter material. Further, the phase and morphological stability of the foam is explored at different conditions such as (i) 50 hours of heat treatment at 650 °C and (ii) 25 days exposed in real-world air at room temperature. It confirms the durability and stability of the foam at different operating conditions.